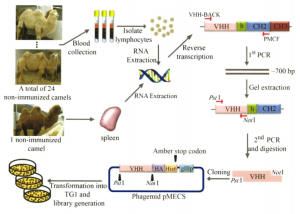
The previous article talked about the first stage of nanobody production, and this article mainly talks about the latter part of antibody production. The specific procedure is as follows:
Coated immune tube:
50ug antigen was added to 2mL PBS and added to the immune tube for overnight incubation at 4℃.
Isolation:
The amplified and purified phage was added to 1mL 3% BSA and incubated for 2h at room temperature. At the same time, 2-3mL 3% BSA was added to the coated immune tubes and incubated at room temperature for 2h.
Antigen and bacteriophage incubation:
The sealed immune tube was washed 3 times with PBS containing 0.01% Tween for 5 minutes each time. The closed phage library was added to the closed immune tube, PBS was added until 2-3mL, and the phage library was incubated at room temperature for 1h.
Cleaning:
After incubating the antigen and phage, the immune tube containing 0.1% Tween was washed 20 times with PBS for 5 minutes each time.
Elution:
1mL 100mM Trimethymime was added to the immune tube, incubated at room temperature for 10 minutes, 1M Tris-HCl was added to neutralize Trimethymime, and the last 1.5mL eluted phage was transferred to a new centrifuge tube. The eluted phage was amplified and purified according to the phage library, and then the screening process was repeated twice after amplification, and the amount of antigen covered by the immune tube was halved successively to obtain the eluted phage after 3 screening. The elution phage can be sequenced by NGS to obtain the candidate nanoantibody DNA sequence library.
ELISA identification:
At the same time, after gradient dilution of the bacteriophages obtained in the previous step, 100ul of each bacteriophage was added to TG1 bacterial solution with OD600nm of 0.5, cultured at 37℃ for 30 minutes, coated with 2x YT culture plate containing ambenomycin, and incubated at 37℃ overnight for the next day to obtain monoclonal colonies. At least 192 single colonies were randomly selected onto 96-well cell culture plates containing 2x YT culture solution and cultured overnight at 37℃ as seed bacteria plates, and the bacterial solution 2ul was re-added to the new 96-well plates (each well containing 200ul 2x YT fresh culture solution). 100ug/ml ammobenzyl) 5 hours later, the auxiliary phage was added into the culture hole, and the final concentration of 50ug/ml kanamycin was added at 37℃ for 30 minutes, and the culture was incubated overnight at 30℃. On the second day, the bacteria solution after overnight culture was centrifuged to obtain the supernatant containing phage.
The overnight coated antigen hole and the BSA coated control hole were sealed with 3% BSA, then the bacteriophage supernatant obtained in the previous step was added, and incubated at room temperature for 1h. After three times of cleaning with PBS containing 0.1% Twain, the phage antibody was added for incubation, and the light absorption value of each well was read at a wavelength of 450nm after color development with TMB. Colonies with large absorbency ratio between antigen-coated pores and corresponding control pores were selected for sequencing (two phage-Elisa analyses independently) to obtain the gene sequence of the nanobodies.
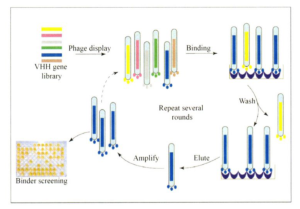
Fig. 1 Antibody screening and identification process
KMD Bioscience provides customized screening system for customers. According to different needs of customers, we provide solid phase screening, solid phase/liquid phase screening and cell screening methods. KMD Bioscience has established a complete and mature phage antibody display technology platform.In addition, KMD Bioscience has rich experience in antibody engineering construction, and can provide three-dimensional antibody upstream and downstream services, including antibody humanization service, human scFv antibody library construction service, human Fab antibody library construction service, human antibody phage library Production service, phosphorylated antibody customization service, antibody affinity maturation service, etc.